Copolymer of Maleic and Acrylic Acid
NANJING FINECHEM HOLDING CO., LTD
General Information
Chemical & Physical Properties
Safety Information
Synthetic Route
| Common Names |
Copolymer of Maleic and Acrylic Acid|furan-2,5-dione,prop-2-enoic acid|Acrylic
acid,polymer with maleic anhydride |
| Structure |
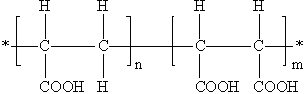 |
| CAS No. |
26677-99-6 |
Boiling Point (℃) |
202ºC at 760 mmHg |
| Molecular Weight |
188.135 |
Melting Point (℃) |
N/A |
| Appearance |
Umber transparent liquid |
Vapor Specific Gravity |
1.18~1.23 |
| HS Code |
3906909090 |
Flash Point (℃) |
N/A |
| Solubility |
Soluble in water, aqueous solution is acidic |
Autoignition Temperature (℃) |
N/A |
| Safety Phrases |
23-26-27-36/37/39-45 |
| RIDADR |
UN 3265 |
| WGK Germany |
N/A |
| Packaging Group |
N/A |
| Hazard Class |
N/A |
|
SYMPTOMS |
PREVENTION |
FIRST AID |
| Inhalation |
Cough. Sore throat. |
Use local exhaust or breathing protection. |
Fresh air, rest. |
| Skin |
Redness. Burning sensation. Itching. |
Protective gloves. |
Remove contaminated clothes. Rinse and then wash skin with water and soap. |
| Eyes |
Redness. Pain. |
Wear safety goggles. |
First rinse with plenty of water for several minutes (remove contact lenses if easily
possible), then refer for medical attention. |
| Ingestion |
Abdominal pain. Nausea. Vomiting. |
Do not eat, drink, or smoke during work. Wash hands before eating. |
Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention. |
Depending on the solvent used in the copolymerization reaction, there are generally two copolymerization
methods.
(1) Solvent polymerization in benzene, toluene, xylene, trimethylbenzene, ethylbenzene, cumene,
butylbenzene or a mixture thereof, white fragile solid polymer can be obtained, the relative molecular
weight is about 4000. Generally, solid copolymers are neutralized with alkali into the form of their
sodium salts to increase the solubility in the copolymer water. 1000 parts of maleic anhydride is
dissolved in 1100 parts (mass ratio) of dried toluene and added to the polymerization reactor equipped
with an electric stirrer, a reflux condenser, and a drip funnel, nitrogen is introduced to remove the
air in the reactor, and the polymerization reactants are protected with nitrogen during the whole
process of the copolymerization reaction. The polymerization reaction temperature is raised to the
toluene reflux temperature (about 123 °C). The measured 80 parts (mass ratio) of dibenzoyl peroxide were
dissolved in the measured 100 parts (mass ratio) of acrylic acid with the removal of the polymerization
inhibitor, and then 700 parts (mass ratio) of dried toluene were added to the acrylic acid peroxide
dibenzoyl mixture and mixed well. Under stirring, add acrylic mixture dropwise to the reactor, maintain
the reflux temperature, control the drop acceleration, and make the acrylic mixture finish within 4h.
Keep the temperature and continue the reaction for 30 min. The reaction copolymer is continuously
precipitated from the reaction mixture, and the heating is stopped after all the copolymers are
precipitated, and nitrogen gas is stopped. Filter the copolymer and dry at 125 °C for 1 h. The dried
acrylic acidmaleic anhydride copolymer was saponified and neutralized with a metered 10% NaOH dilute
solution to form an aqueous copolymer solution with a total solid content of 25%~30%. The aqueous
solution is light yellow and the average relative molecular mass of the copolymer is about 4000.
(2) Aqueous solution polymerization If water is used as the polymerization medium, maleic anhydride must
first be converted into its sodium salt form with alkali to increase its solubility in cold water.
Maleic anhydride can also be added to water to heat up to 90~100 °C, so that it is dissolved in hot
water, and then copolymerized, so that the co-obtained product is directly dissolved in water, and the
aqueous solution is slightly yellow. The metered maleic anhydride is neutralized with isomolar sodium
hydroxide to make the sodium of maleic anhydride Salt. Add an appropriate amount of deionized water to
the polymerization reactor equipped with an electric stirrer, a drop funnel and a reflux condenser,
dissolve the metered sodium maleic anhydride salt in the water in the reactor, and raise the reaction
temperature to 95~100 °C under stirring. Take the metered ammonium persulfate and add It is put into the
metered acrylic acid, and the dilute solution of acrylic acid ammonium persulfate is prepared with an
appropriate amount of deionized water, and placed in the drop funnel on the reactor. When the reaction
temperature in the reactor rises to the required temperature, start to add acrylic mixture dropwise,
control the drop acceleration, add it within about 4h, after adding the acrylic mixture, maintain the
reaction temperature to continue the reaction for 1h, stop the reaction, and obtain a light yellow to
colorless copolymer aqueous solution.
Frequently Asked Questions
What is Copolymer of Maleic and Acrylic Acid(MA/AA)?
Maleic acid-acrylic acid copolymer (MA/AA) is a low-molecular-weight polyelectrolyte, which is prepared
by copolymerization of maleic acid and acrylic acid in a certain proportion. MA/AA has a strong
dispersing effect on carbonate, etc. It has high stability, and it can be used under harsh conditions
such as 300 ℃ high temperature, and has good compatibility and synergistic effect with other water
treatment chemicals. It has a good inhibitory effect on the formation of scale including phosphate. Due
to its excellent anti-scale performance and high temperature resistance, MA/AA is widely used in
low-pressure boilers, central heating, central air-conditioning and various circulating cooling water
systems. MA/AA can also be used as a chelating dispersant in the textile printing and dyeing
industry.
The application range of Copolymer of Maleic and Acrylic Acid(MA/AA)?
MA/AA can be used in high temperature circulating water system or low pressure boiler and distillation
system. It is used in combination with other organic phosphates, and the dosage is generally
2-10mg/L.
The amount of MA/AA used as textile printing and dyeing auxiliaries and builders is determined by
experiments.