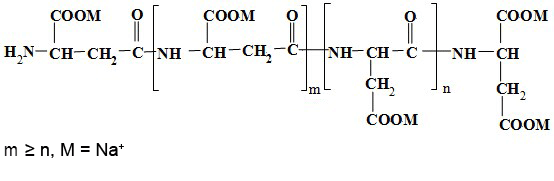Frequently Asked Questions
What is Sodium Salt of Polyaspartic Acid?
Sodium Salt of Polyaspartic Acid is a biomimetic synthetic water-soluble polymer substance, which has
the characteristics of phosphorus-free, non-toxic, pollution-free and completely biodegradable. It is an
internationally recognized "green chemical". Due to the structural characteristics of polyaspartic acid
containing active groups such as peptide bonds and carboxyl groups, it has extremely strong chelating,
dispersing, and adsorption functions, and has excellent compatibility. This product is widely used as a
fertilizer synergist, as a scale and corrosion inhibitor in industrial cooling circulating water,
reverse osmosis water, oilfield reinjection water, metal cutting fluid, boiler and steam pipeline and
other water treatment fields. And it can be used as a dispersant in papermaking, printing and dyeing,
and washing industries, and it is also used in the field of daily chemicals.
What is Sodium Salt of Polyaspartic Acid used for?
As a new type of green water treatment agent, Polyaspartic Acid is applied to the field of industrial
circulating cooling water treatment. Polyaspartic Acid can chelate calcium, magnesium, copper, iron and
other multivalent metal ions, especially can change the crystal structure of calcium salt to form soft
scale, which can be used in water treatment fields such as industrial circulating water, boiler water,
reverse osmosis water, oilfield water and seawater desalination. it performs well in systems with high
hardness, high alkalinity, high pH value, and high concentration multiples. The scale inhibition effect
of PASP is better than that of commonly used phosphine-containing scale inhibitors. PASP and PBTCA have
a synergistic effect after compounding.
As metal corrosion inhibitor, Polyaspartic Acid can get better corrosion inhibition effect when the pH
is above 10. When the pH is between 8 and 9, the lower concentration of Polyaspartic Acid has better
corrosion inhibition effect in seawater. Polyaspartic Acid is compounded with organic phosphorus, sodium
tungstate, quaternary ammonium salt, zinc salt, molybdate, oxidized starch, etc. to achieve better
corrosion inhibition effect.
Application of Polyaspartic Acid in agricultural field.
Polyaspartic Acid itself is not a fertilizer, but it can be used as a fertilizer synergist. Polyaspartic
Acid has a chelating effect on metal ions. Polyaspartic Acid with a certain molecular weight can enrich
nitrogen, phosphorus, potassium and trace elements to supply plants, so that plants can use fertilizers
more effectively and improve the yield and quality of crops . The addition of Polyaspartic Acid can
increase crop yield and improve soil quality.
Application of Polyaspartic Acid in the field of hygiene.
As a new type of high polymer, Polyaspartic Acid can react with cross-linking agent to further
synthesize super water-absorbing agent. This water-absorbing agent is easy to degrade and can be used as
fertilizer after degradation without any pollution to the environment. The features meet the
requirements of sanitary products for super absorbent resin.
Polyaspartic Acid can be used as a cleaning agent, not only can replace phosphorus-containing
detergents, but also can be added to dishwashing detergents because it is safe, non-toxic and
non-irritating. And its effect is better than water and polycarboxylate cleaning effect. It can also be
used as drilling fluid viscosity reducer, coal water slurry dispersant, detergent synergist, etc., and
can also be applied to cosmetics and health products.

.jpg)