

| Item | Index |
|---|---|
| Appearance | Light yellow to umber transparent liquid |
| Active content (as salt) | 35.0min |
| Active content (as acid) | 25.0min |
| PH (1% water solution) | 10.0-11.0 |
| Density (20℃) g/cm3 | 1.20min |
| Fe (Fe2+),mg/L | 20max |
| Ca sequestration (mg CaCO3/g) | 250min |
| Total phosphorus (asPO43- )% | 20.0min |
| Packing | 200L plastic drum,IBC(1000L),customers’ requirement. |
|---|---|
| Storage | 20℃, 2 years. |
| Shipping | Room temperature in China; may vary elsewhere |
Tel: 0086 25 52397805
Email: sales2@nj-finechem.com

| Common Names | Sodium Salt of Triethylene-tetramine Hexmethanephonic Acid|TETHMP | ||
|---|---|---|---|
| Structure | 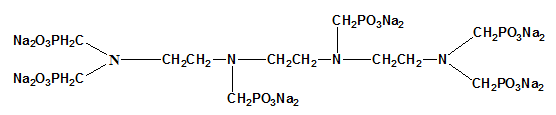 |
||
| CAS No. | N/A | Boiling Point (℃) | N/A |
| Molecular Weight | 974 | Melting Point (℃) | N/A |
| Appearance | Light yellow to umber transparent liquid | Vapor Specific Gravity | N/A |
| HS Code | N/A | Flash Point (℃) | N/A |
| Solubility | N/A | Autoignition Temperature (℃) | N/A |
| Safety Phrases | N/A | ||
|---|---|---|---|
| RIDADR | N/A | ||
| WGK Germany | N/A | ||
| Packaging Group | N/A | ||
| Hazard Class | N/A | ||
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Skin | Redness. Burning sensation. Itching. | Protective gloves. | Remove contaminated clothes. Rinse and then wash skin with water and soap. |
| Eyes | Redness. Pain. | Wear safety goggles. | First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention. |